Xe is the central atom:
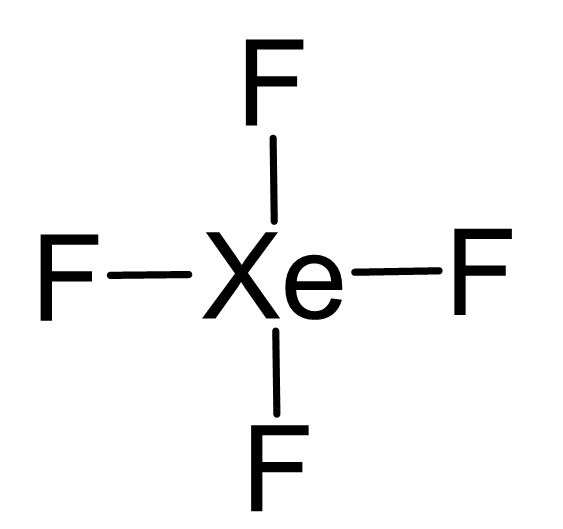
There are 4×7 + 8 = 36 electrons and 8 are taken to make 4 covalent bonds. Each fluorine takes 3 lone pairs, so there are 36 – (8+4×6) = 4 electrons left which go to Xe as 2 lone pairs:
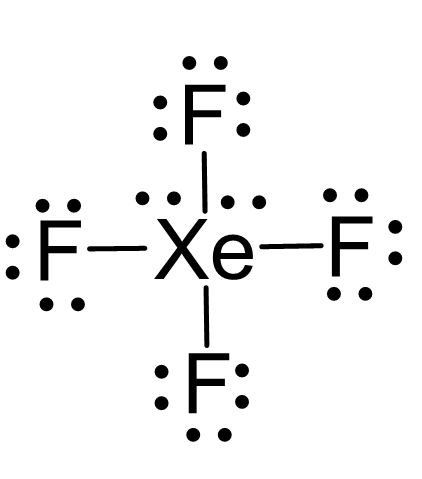
There are 4 atoms and 2 lone pairs on the central atom, therefore, the steric number is 6 and the electron geometry is octahedral and the molecular geometry is square planar:
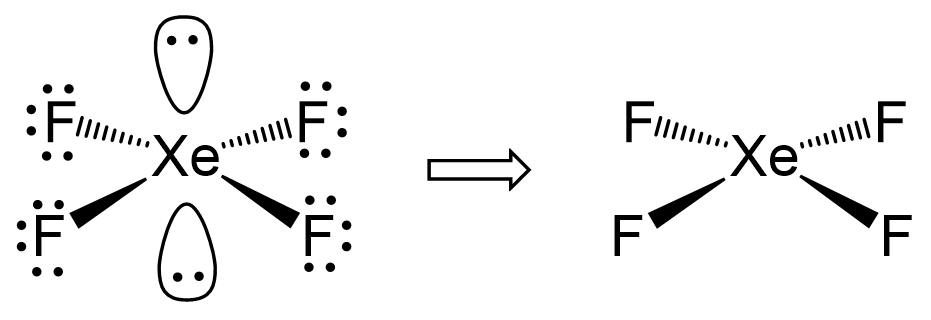
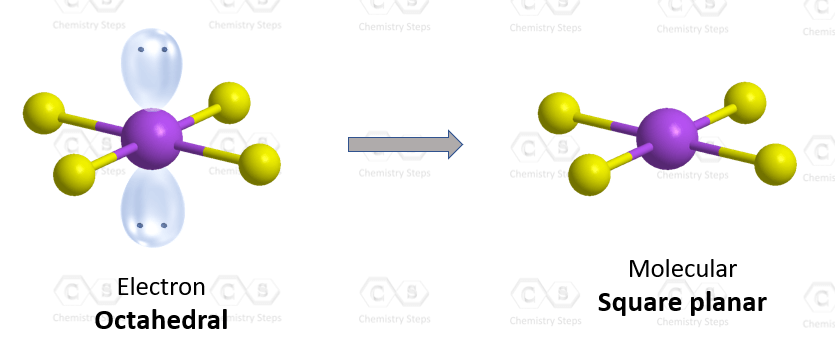
There are 6 units (atoms and lone pairs) on the central atom, and to accommodate them, it needs 6 orbitals which is achieved through sp3d2 hybridization.
