Let’s explain what mixture is in simple words without going into formal language. A mixture is something that is made by mixing two or more different things. Mixtures are everywhere we look. For example, you are sitting on the beach having a cup of coffee. Now, the sea is a mixture, the sand is a mixture, the coffee is a mixture, and any other drink you are going to have is also a mixture. The air you are breathing is a mixture as it consists of oxygen, nitrogen, carbon dioxide, and other gases in small quantities.
Now, not all the mixtures are identical, in that, for some, we can see the individual components like in a salad, and for others, like a cup of tea, we can’t see what it consists of. Because of this, mixtures are classified as homogeneous and heterogeneous. Homogenous mixtures are also called solutions, and these are characterized by evenly distributed components throughout the mixture. For example, a spoonful of sugar dissolved in water makes a solution where sugar is the solute and water is the solvent. In general, the component that is present in greater quantity is classified as the solvent and the solute is the one present in less amount.
In a heterogeneous mixture, on the other hand, the components are visibly distinguishable and not evenly distributed throughout.
Let’s put a flow chart showing how matter is classified into pure substances and mixtures:
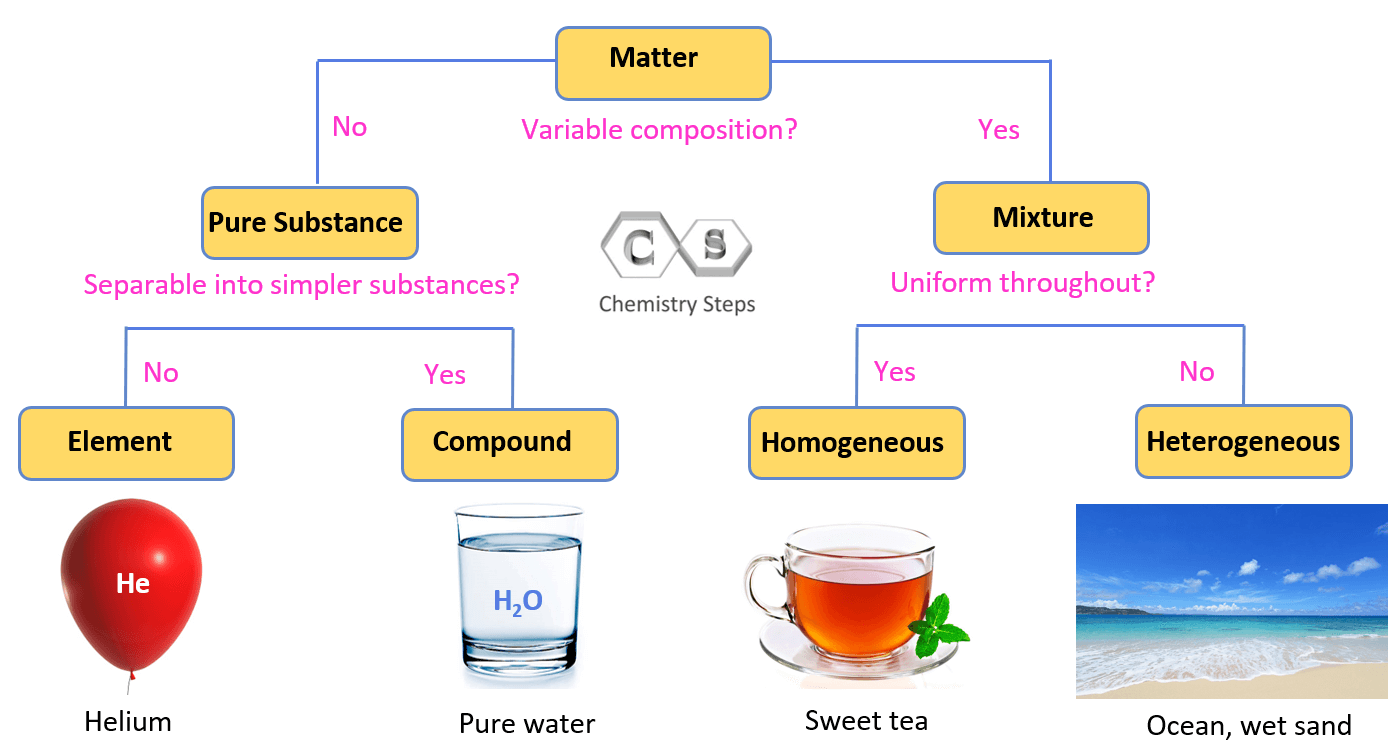
Generalizing what we discussed, we can say that a homogeneous mixture (or solution) has no visible boundaries as it has the same composition throughout. Although most homogenous mixtures are liquid and it is a common perception that solutions can only be liquid, you need to know that solutions can be in a solid gas state as well. For example, many alloys, polymer blends, and ceramics are solid solutions.
A heterogeneous mixture does not have a uniform composition and a boundary is visible among the components. Now, just because we do not see this boundary or boundaries, does not mean that it is a homogenous mixture. Sometimes, special apparatus is needed to spot them. For example, milk and blood are heterogenous mixtures even though we cannot see their components with the naked eye. Blood contains many components such as proteins, platelets, white blood cells, red blood cells and etc. that can be seen under a microscope. Other common examples of heterogeneous mixtures are most dishes, salads, yogurts, rocks, and minerals.

What is Considered Not a Mixture?
So, on the left side of the chart, we have examples of not mixtures. Because we mentioned that most of the matter that we see around is a mixture, you may be wondering what is not a mixture then.
Well, we mentioned that the air is a mixture, but if we take the oxygen gas by itself, it is not a mixture anymore. It is a pure substance, and more specifically it is a compound with the formula of O2. Other examples of pure substances would be hydrogen, water, and table salt (sodium chloride, NaCl) because they are all made up of only one component and their composition does not change from one sample to another.
Elements and Compounds
Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Another example can be water where two hydrogen atoms are connected to an oxygen atom.
Any monoatomic element that you can think of or find in the periodic table would be an example of an element.
Check this 36-question, Multiple-Choice Quiz on Matter including questions on mixtures, compounds, molecules, and more.
Free

Quiz: Matter, Chemical and Physical Properties
1.
The law of constant composition applies to ________.
A) solutions
B) heterogeneous mixtures
C) compounds
D) homogeneous mixtures
E) solids
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
2.
What is formed when sand, sugar, and water are combined?
A) a compound
B) a heterogeneous mixture
C) a pure substance
D) a homogeneous mixture
E) a solution
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
3.
Dissolving a small amount of food dye in water results in a ________.
A) pure substance
B) heterogeneous mixture
C) homogeneous mixture
D) compound
E) solid
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
4.
Filtering or decanting can often be used to separate the following into its components.
A) elements
B) compounds
C) homogeneous mixture
D) heterogeneous mixture
E) pure substances
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
5.
Which states of matter can be compressed significantly?
A) solids only
B) liquids only
C) gases only
D) liquids and solids
E) solids and gases
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
6.
Varying compositions are possible for…?
A) element
B) compounds
C) homogeneous mixture
D) heterogeneous mixture
E) homogeneous and heterogeneous mixtures
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
7.
A uniform matter that cannot be separated into other substances by physical means, is ….
A) a homogeneous mixture
B) a heterogeneous mixture
C) a compound
D) either an element or a compound
E) an element
F) a solution
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
8.
What is a characteristic feature of an element?
A) it cannot be part of a heterogeneous mixture
B) it cannot be broken into other elements by chemical means
C) it cannot be part of a homogeneous mixture
D) it can only interact with other elements to form compounds
E) it is not always a pure substance
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
9.
The term “solution” can be applied to:
A) homogeneous mixtures
B) compounds
C) elements
D) liquids
E) substances
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
10.
Which of the following is NOT an example of a chemical reaction?
A) the reaction of Mg with hydrochloric acid
B) a firework show
C) the burning of plastic
D) the sublimation of iodine
E) the rusting of iron
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
12.
Brass is a:
A) element
B) compound
C) homogeneous mixture
D) heterogeneous mixture
E) mixture of carbon and oxygen
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
13.
Potassium hydroxide is a:
A) homogeneous mixture
B) atom
C) element
D) heterogeneous mixture
E) compound
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
14.
The term substance is applicable to:
A) any mixture
B) compounds
C) elements
D) elements and compounds
E) elements, compounds, and mixtures
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
15.
Which one of the following would be classified as a heterogeneous mixture?
A) salt water
B) potassium nitrate solution
C) mix of sugar and vanilla powder
D) brass
E) a salt solution
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
16.
Which of the following is a compound?
A) ammonia
B) hydrogen gas
C) betadine
D) ketchup
E) nitrogen gas
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
17.
H2SO3 is:
A) a mixture
B) a compound
C) an element
D) a salt
E) a molecule and a compound
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
18.
P4 is:
A) a mixture
B) a compound
C) an element
D) a molecule
E) a molecule and a compound
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
19.
What best describes a substance that can’t be chemically broken down into simpler substances?
A) an element
B) a homogeneous mixture
C) a heterogeneous mixture
D) a compound
E) a solution
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
20.
Separating a solid from a liquid by pouring off the liquid is called:
A) Distillation
B) Evaporation
C) Filtration
D) Decanting
E) Lyophilization
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
21.
Decanting is:
A) boiling a mixture of liquids to separate them based on their different boiling points
B) dissolving a solid into a liquid
C) separating a solid from a liquid by pouring off the liquid
D) pouring a mixture of a solid and liquids through a filter paper to separate them
E) fusing two solids together by heating them
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
22.
Distillation is:
A) boiling a mixture of liquids to separate them based on their different boiling points
B) dissolving a solid into a liquid
C) separating a solid from a liquid by pouring off the liquid
D) pouring a mixture of a solid and liquids through a filter paper to separate them
E) shaking a mixture and letting the solid to accumulate on the bottom
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
23.
Filtration is
A) boiling a mixture of liquids to separate them based on their different boiling points
B) dissolving a solid into a liquid.
C) separating a solid from a liquid by pouring off the liquid.
D) pouring a mixture of a solid and liquids through a filter paper to separate them
E) heating a mixture of two solids to fuse them together.
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
24.
The following are intensive properties except:
A) mass
B) density
C) melting point
D) temperature
E) freezing point
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
25.
None of the following is an extensive property except:
A) boiling point
B) density
C) volume
D) temperature
E) freezing point
answer
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.
Solution
This content is available to registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the Answers and Solutions for all the Practice Problems, Quizzes, and the powerful set of General Chemistry 1 and 2 Summary Study Guides.



