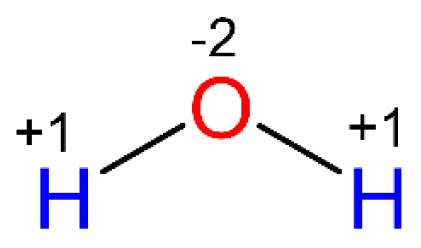
Molecular, Ionic, and Net Ionic Equations
All the equations where the reactants and products are shown as molecules with complete chemical formulas are called molecular equations. This, essentially, is what we use for most reactions. For example: 2K3PO4(aq) + 3BaCl2(aq) → Ba3(PO4)2(s) + 6KCl(aq) However, we … Read more