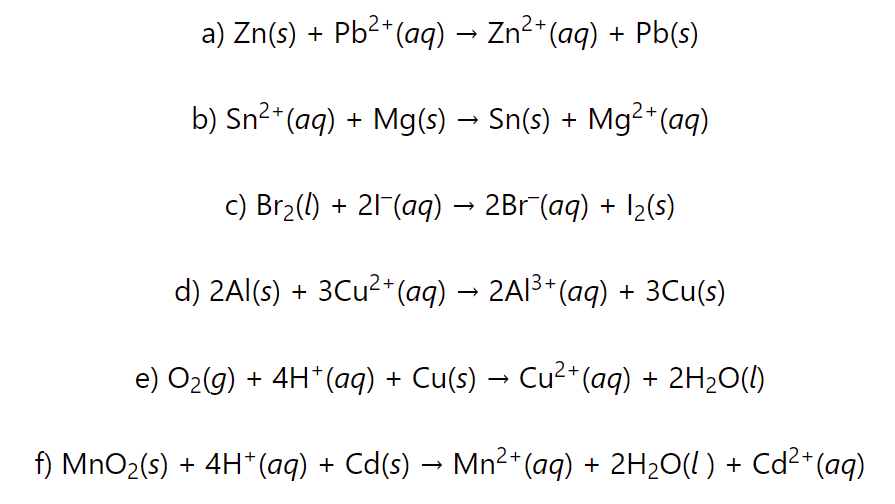Calculate the Cell Potential for Each Redox Reaction
Calculate the cell potential for each redox reaction and determine if it is spontanesous or not: a) Zn(s) + Pb2+(aq) → Zn2+(aq) + Pb(s) b) 2Cl–(aq) + Pb2+ (aq) → Cl2(l) + Pb(s) c) Cd(s) + Pb2+(aq) → Cd2+(aq) + Pb(s) … Read more